Posts by Rick Pollock
Stacie Lindsey
President, Herriman, Utah Stacie’s brother, Mark Clements, was diagnosed in 2005 and ultimately passed away from Cholangiocarcinoma on January 19, 2007. Stacie advocated for her brother, extensively researched treatment options and began networking with other patients, researchers and healthcare professionals. From this experience, the Cholangiocarcinoma Foundation was born. Stacie is a founding member and has served…
Read MoreModeling Cholangiocarcinoma
Modeling Cholangiocarcinoma Webinar from Cholangiocarcinoma Foundation on Vimeo. “Like all cancers, cholangiocarcinoma is a genetic disease. I will present our effort to comprehensively characterize the genetic landscape of cholangiocarcinoma and to use this information to develop genetically representative models of this disease that will serve as instrumental tools in our search for novel therapies. ” –…
Read MoreUnderstanding Cancer in the Age of Genomics
Understanding Cancer in the Age of Genomics from Cholangiocarcinoma Foundation on Vimeo. Presented by Dr. C. Jimmy Lin MD, PhD, MHS. About Dr. Lin and his work in genomics.
Read MoreNew Intrahepatic Cholangiocarcinoma Clinical Trial
New Intrahepatic Cholangiocarcinoma Clinical Trial from Cholangiocarcinoma Foundation on Vimeo. Intrahepatic cholangiocarcinoma affect approximately 2000 people a year in the US. Many patients are not able to have an operation to remove the bile duct cancer. We are preparing to enroll patients in a clinical trial which includes patients who can have an operation and…
Read MoreFeeling Guilty for Having Cancer
Feeling Guilty for Having Cancer from Cholangiocarcinoma Foundation on Vimeo. No one asks for cancer, but when the diagnosis is confirmed some patients feel guilty for being sick! In this informative webinar, Dr. Geret Giles discusses the phenomena of Patient Guilt and what we can do about it–for ourselves and for the ones we love.
Read MoreGrant Recipient Reports on Research Progress
Grant Recipient Reports on Research Progress from Cholangiocarcinoma Foundation on Vimeo. Surbpong Tanasanvimon received a LIFe recipient grant from The Cholangiocarcinoma Foundation in 2012 this grant was awarded through the Conquer Cancer Foundation (which is the non-profit arm of ASCO). He recently had the opportunity to present his research at the 2013 GI Symposium.
Read MoreCEACAM6 as a Biological Marker and Prognostic Factor for Biliary Malignancy
In 2011, The Cholangiocarcinoma Foundation funded two grants through the Conquer Cancer Foundation: the Young Investigator Award and the Long Term International Fellowship Award. The Young Investigator Award was presented to Dr. Flavio Rocha.
Read MoreAsk Dr. Giles: I’m angry at my parents’ friends who have done nothing to help them
Lisa asks: Dr. Giles, My father is close to the end with this disease after about 6 months of being sick. I am so grief stricken and angry. The grief is overwhelming at times and I think things like, it would be better if we all died the minute we were born rather than live…
Read MoreAsk Dr. Giles: How do I move past the “what if’s”?
Tiffany asks: Dr. Giles: I’m fighting CC. I have a friend named Liza that is also fighting cancer (liver). She and I were friends before cancer. She is only 24, and I’m 30. Liza isn’t doing good. In fact she is about to die. How to I protect myself while show support to her family?…
Read MoreCaregivers and the emotions of caring for a patient with a terminal illness
Does caring for your loved one leave you feeling overwhelmed with stress, anxiety, depression, and fear… (there are a few hiccups during the first 10-15 seconds, they do not persist through the entire video. we apologize for the inconvenience.)
Read MorePatient Registry
Dr. Lewis Roberts from The Mayo Clinical will highlight details about the patient registry at The Mayo Clinic, and how this registry can assist with efforts in advancing research of bile duct cancer.
Read MorePhase II Study Of Intrahepatic Arterial Injection of 90-Y Glass Microspheres As First-Line Treatment
This is a trial using intra-arterial injection of radioactive glass microspheres as first-line treatment for intrahepatic cholangiocarcinoma…
Read MoreAsk Dr. Giles: How do we even begin to deal with this?
Cindy asks: My mother was diagnosed with cholangiocarcinoma in June 2007. She has surgery to remove a tumor the size of an orange in the right lobe of her liver. She then underwent 9 chemo treatments. In June of 2011 she was diagnosed with stage 4 cholangiocarcinoma that metastized to her lungs and lymph nodes,…
Read MoreAsk Dr. Giles: My niece doesn’t know the extent of her cancer, should we tell her
A Concerned Aunt asks: Hi, My 29 year old niece was diagnosed with cholangiocarcinoma about 2 weeks now. The doctors here in PH told us it already on stage 4 and surgical procedure is no longer an option. My niece is all in high spirits though we see her health deteriorate day by day. My…
Read MoreBiliary Tract Diseases
The Cholangiocarcinoma Foundation, in partnership with the Primary Schlerosing Cholangitis Foundation, hosted this webinar focused on biliary…
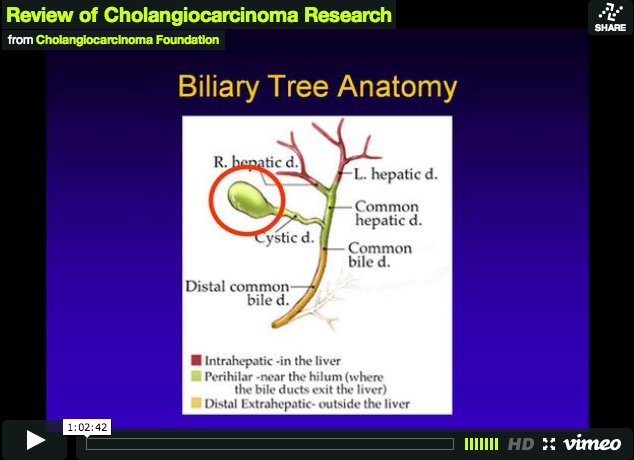
Read MoreReview of Cholangiocarcinoma Research
Dr. Ghassan K. Abou-Alfa from Memorial Sloan-Kettering Cancer Center lead the discussion, which included noteworthy updates from the 2011…
Read MoreInterview with OncUViewTV
From ASCO 2011 — A discussion with Sara Hinkley, President of The Cholangiocarcinoma Foundation about the foundation and bile duct cancer.
Read MoreAsk Dr. Giles: What emotional support can we give our brother
Angela asks: Our offers to help our brother-in-law and his family seem empty but very much appreciated by them. We live an hour away, so its difficult to assist with coordinating meals, errands, and pick-up/drop-off for their daughter. We’ve hosted mass intentions for him, but feel that’s about all we can do. What emotional support…
Read MoreAsk Dr. Giles: My husband glares at me and doesn’t speak to me as if he’s angry.
Charlotte asks: I am a 67 year old female with CC diagnosed in Jan 2010 and doing very well from a cancer perspective. I am having a lot of hip pain requiring a walker so my husband has to help with carrying laundry up and down stairs and going to the grocery. He says he…
Read MoreSpotlight Webinar Series: 2011 Review of Chemotherapy Treatment in Bile Duct Cancer
As part of our ongoing educational series, The Cholangiocarcinoma Foundation is proud to present a review of chemotherapy treatment for… posted: Apr. 12, 2011
Read MoreAsk Dr. Giles: I’m scared that something will happen to my husband and I’m not ready
Missy asks: My husband Kevin has cc and won’t do chemo any more. I want him to do chemo and the natural healing stuff together because I feel go at it with both. He tells me chemo isn’t a cure. I’m honestly not convinced that naturally is. My husband is a very strong man but…
Read MoreAsk Dr. Giles: I was involved in the beginning, but now I feel like a spectator
Courtney asks: My Father has survived one year so far after surgery for Klatskins CC. He has had non stop complications ranging from infections, to bleeding ulcers, to anorexia type behavior…I am 7 hours away and my step mother is his main caretaker. It is very complicated and confusing to try to keep up with…
Read MoreAsk Dr. Giles: My cancer is gone, but I don’t know how to regain my joie de vie.
Donna asks: In late 2007 I was diagnosed with cancer. Originally it was described as adenocarcinoma, stage 4, terminal. I had been going to the DR for 4 years complaining of pain and/or discomfort in the upper abdomen. Lots of blood work was done but nothing was identified. In August of 2007 I say a…
Read MoreAsk Dr. Giles: I worry that I may be pushing my grandmother too far
Courtney asks: My grandmother has cc! I was raised by my grandparents, I just recently lost my mother of a heart attack she was 44. My grandmother is such a strong woman and has had surgery, right liver resection portion of left all that goes with that. She did complete i round of radation and…
Read MoreAsk Dr. Giles: I’m determined to get help with my grief, but I’m just so tired and confused.
Michael asks: My name is Michael and on September 28 of this year I lost my grandmother and best friend to bile duct cancer after a 6 week battle. I’ve been posting on the discussion board and they recommended you as a good person to contact with addtional questions. I am currently seeing a one…
Read MoreWebinar: Dealing with the Emotions of Diagnosis
” . . . the test results are back and there are tumors on your liver . . .” With those chilling words, the emotional and physical battle…
Read MoreBranching Out: How To Establish a Local Cholangiocarcinoma Support Group
The Cholangiocarcinoma Foundation is pleased to introduce an informational series focused on helping you get involved in with developing…
Read MoreSpotlight on Young Investigator Award and 2010 Review
Back in September 2010, The Cholangiocarcinoma Foundation announced that it was partnering with the American Society of Clinical Oncology…
Read MoreAsk Dr. Giles: I worry I’m not showing friends and family appreciation.
Lina asks: Even though I have the most amazing awesome support of friends and family who are giving me all the love and support, emotionally and financially, I wonder why I feel depressed at times and simply want to burst into tear. Am I doing enough chemo to tell my friends and family that I…
Read MoreAsk Dr. Giles: How do we make the most of the time we have left?
Mary Anne asks: My 79yo dad was just dx with cholangiocarcinoma. Except for the past 2 weeks where he became ill leading to the dx, he has been extremely active(bike riding, bowling,handyman etc). The recent illness and dx have taken the wind out of his sails. I realize he needs time to adjust and absorb…
Read More2010 Review of Controversies in Surgical Treatment
As part of our ongoing educational series, The Cholangiocarcinoma Foundation invites you to join us for the upcoming web-based seminar featuring…
Read MoreAsk Dr. Giles:How to cope with BiPolar/Narcissistic husband with cholangiocarcinoma.
Tina asks: My Husband was diagnosed in June ’09 with cholangiocarcinoma that is unresectable, at that time he was told that his prognosis was 9-12 months. He completed chemo (gemzar and cisplatin) in the fall and a CT was completed at that time (Dec), which his Oncologist had stated that he was staying with his…
Read MoreAsk Dr. Giles: Mom died of cholangiocarcinoma, worried she might have it too
Leanne asks Hello, well my mother was diagnosed with CC in jan 09 and was gone by march 09… i have a history of lupus plus and have had pain for a long time in the liver region, they suspect its the bile ducts and im petrified… how do i deal with these doctors who…
Read MoreAsk Dr. Giles: I’m overwhelmed.
Beth asks: Hello, I am a 34 yr old caregiver to my husband who is also 34. Recently there is talk from our Dr.s that they might not be able to continue treatment. I’m sad. We have a 5 year old daughter and I don’t know how I will move on. My husband is obviously…
Read MoreAsk Dr. Giles: I’m not ready to let go of mom, is there anything I can do
Corina asks: Dear Dr. Giles, Nov.09 I took my mom with excruciating abdominal pains to ER. She had an ERCP and a plastic stent was inserted; while still in hospital in Dec.09 another ERCP was done to crash the stone- gallblader removed 15yrs ago- this time I was told a 2.7cm tumor, diagnosis:DISTAL BILE DUCT…
Read MoreAsk Dr. Giles: I don’t know what to expect
Anjie asks: My brother was recently given his “expiration” date and it is really the elephant in the room disease. It is very difficult to talk about which is the way our family has always been. My question is more medical in nature, but don’t know who to go to. He is beginning to be…
Read MoreAsk Dr. Giles: My mom’s afraid of chemotherapy
Benjamin asks: My mother was just dx with Cholangiocarcinoma 3 weeks ago and my family and I are trying to get our heads around it. My Mother is 54 and wasn’t feeling good since December, she started having abdominal pain and losing weight. My mother is scared about the pain and is on the fence…
Read MoreAsk Dr. Giles: Should I be concerned about his stress at work affecting his health
Jeff asks: My partner was diagnosed with CC in early February and is undergoing the Gem/Cis Chemo Treatment. He is doing OK so far on that. However, he is back to work and is very stressed. Should I be concerned about his stress at work affecting his health? Dear Jeff, I am going to assume…
Read MoreAsk Dr. Giles: She seems to be getting better, but also distant
Kate asks: Hi. My partner has unexpectedly gotten through a whipples, and has now completed a round of chemo. Great, but she has now signed a non-intervention document, and is emptying the house of her belongings. Our relationship is great, and I accept her right and choice, and while I am finding the emptying on…
Read MoreAsk Dr. Giles: I just want to connect with someone like me who survived this
Randi asks: First let me say how sorry I am about the loss of your friend. I am a 54 year old woman who was diagnosed with CC after having an ERCP in Nov 2009. I had a Whipple procedure December 15th, 09 (just 4 weeks ago) and the good news was that the cancer…
Read MoreClinical Trials 101
Have you found the clinical trial process confusing? Or are you looking for additional information on the available bile duct cancer clinical… posted: Feb. 19, 2010
Read MoreAsk Dr. Giles: How can I be dying when I feel so good?
Cheryl asks: How can I be dying when I feel so good? Diagnosed with cholangiocarcinoma, Sept. 11 2009, have had Sir-Sphere treatment and many chemo treatments. Sometimes I feel ill, but generally I feel normal, happy and thankful. If I felt bad maybe I could come to grips with this disease that is killing me.…
Read MoreAsk Dr. Giles: How can I lovingly but firmly ask mom to back off?
anonymous writes: I have cancer and my mother is caring for me. Actually, I hate to sound ungrateful, but she is smothering me. She is not allowing me to breathe. I am a more introverted person and need time alone to marshall my resources against this cancer and she is always tap, tap, tapping at…
Read MoreAsk Dr. Giles: What can I do ease my pain and devastation?
Rochelle writes: I lost my husband two days ago, he was my absolute everything, we just recently moved to casper and I have no support. I do not think that I can cope with his passing he was my best friend, my lover, and my husband, he was the love of my life and I…
Read MoreAsk Dr. Giles: I’m the caregiver, and I feel overwhelmed.
Melanie writes: Hey Doc, I’m the caregiver, and he’s so sick..so miserable. Nobody really seems to know anything about this disease. As the care giver? S.O., I’m overwhelmed. I think I’m starting to have panic attacks myself. I’ve been taking some homeopathic stuff for a week or so, seems to help a little, sometimes. I…
Read MoreAsk Dr. Giles: I feel selfish for wanting to care for my sister’s kids
a Concerned Sister writes: I would welcome any advice on this situation. My sister is due for the big surgery very soon, she was told the longest she will last with everything going well would be 5 years max. Her tumor has spread from the ducts to a good portion of her liver. She is…
Read MoreAsk Dr. Giles: Book recommendations for preparing my famly
George writes: Can you recommend a book that will guide me through preparing to succomb to cancer, one that will list some steps I need to do for my family. For example I’m getting around to finally doing a will etc. Thanks George, My friend Marion shared this link to a resource of the National…
Read MoreAsk Dr. Giles: How do I help my husband deal with my diagnosis?
Carol writes: I have been dx w/cholangiocarcinoma. I’m 66 and about 3 wk. I have always been healthy and began losing a lot of weight. Went to my Dr, had tests, finally biopsy and dx. At first it was believed they could resection but now find it can’t be done. My husband and I have…
Read MoreAsk Dr. Giles: How to come to grips with my breast cancer and my dad’s diagnosis
Claudia writes: My father was diagnosed 3 weeks ago with cholangiocarcinoma, we did not know the extent of the cancer and surgery was what was recommended. They removed gall bladder, 60% of liver and reconnected the intestine to liver. Now he has major complications, a leak somewhere and he is very weak. I am having…
Read MoreAsk Dr. Giles: How can I make the very most out of this chance to be with her
Vicki writes: My 89 yr old grandmother has been diagnosed w/ this. I am going to Ohio (I live in NE) to spend 2 weeks with her, knowing it will probably be my last time. How do i handle this? I do not want to spend the whole time freaking out because she is dying,…
Read MoreAsk Dr. Giles: I am filled with depression, anxiety and feel distraught every minute.
Sophie writes: Dear Dr. Giles, I am a 62 year old woman with cc and 75% of my liver metastisized. I have had to be so strong all my life and thought I was, but now I don’t believe I am. I live by myself, and my mind is so dark all the time. I…
Read MoreAsk Dr. Giles: I’m worried that the stress of caring for our mother is causing resentment.
Marg writes: Our 87 year old mother was diagnosed with advanced CC in early March. The specialist told us that she had 2-4 weeks to live. With this prognosis, my three sisters and I decided that we would take turns caring for Mom at her home where she lives with my father. There was also…
Read MoreAsk Dr. Giles: I’m an emotional mess that’s angry and scared.
Tonia writes: Hi, In short my Grandmother was diagnosed with bile duct Cancer “officially” on the 13th. We had a strong suspicion on the 3rd when her coloring turned yellow but hung onto hope that maybe it wasn’t. She had the bypass surgery on the 13th and the Cancer was found in her lymph nodes…
Read MoreAsk Dr. Giles: I’m angry at my mom’s doctor for not finding this earlier
Belinda writes: My mother was going to her doctor for two years she was telling her how bad her stomache hurt, she lost 18lbs could hardly eat the pain was so bad, this christmas she blacked out and couldnt breath,at the hospital they found 15 tumors in her liver and told her she had cancer,…
Read MoreAsk Dr. Giles: I feel guilty for retiring early due to this illness.
Lisa writes: I’m feeling guilty about retiring early due to health reasons. I don’t feel *that* bad, but I’m finding it difficult to keep juggling work, family and health issues. I’m only 46, a single parent, and still supporting my 3 kids. By cutting all my expenses to the bone, I think we can make…
Read MoreAsk Dr. Giles: Any tips or suggestions on how to recover faster?
Eric writes: Dear Dr. Giles, first of all, i am so sorry for the loss of your best friend. I am a 14 year-old boy and my mom passed away of cc. in July 08 at 40yrs old. everything was so sudden, 2 months before she was fine and active with just a little stomach…
Read MoreAsk Dr. Giles: Should I do a motivational recording or video for my wife?
Jeff writes: Do you think it would be appropriate for me to do like a motivational audio or video for my wife,that she could listen or watch after my demise. My thought is when she feels really down and out and needs reassurance and some motivation woud it be a good thing or would it…
Read MoreAsk Dr. Giles: My husband’s adult children don’t get it.
Joan writes: My husband & I have been married 7 years and he has 5 grown kids (27-36 yrs old) from a previous marriage. Recently, after being diagnosed (8/07) with cholangiocarcinoma we retired & moved to from NJ where all his kids live to our “dream” island of Hilton Head, SC. My husband is now…
Read MoreAsk Dr. Giles: How do I go on?
Charlene writes: My husband died 1 week and 1 day ago. We fought this cancer for 3 long years. How do I go on? My soul is dead. All I feel is agony every waking moment. I don’t know how to verbalize what is happening to me. My family just tells me to be strong,…
Read MoreAsk Dr. Giles: Should I ask hospice about counseling for my 17 year-old son?
Carol writes: Our family is meeting with Hospice and trying to get things in order for Charlie as there are no more medical interventions to try. He is jaundiced, not eating and weak. My question is about our son Ben, age 17. He is having a great deal of trouble sleeping. Do you think asking…
Read MoreAsk Dr. Giles: My husband passed very quickly, we didn’t have a chance to say that last goodbye.
Darla writes: My husband passed away from Cholangiocarcinoma after only 7 short weeks. He was not even definitively diagnosed until 1 week before he died. At that time he was given 6 months without any treatment. A week later we were told he only had a short time left, possibly up to a month. He…
Read MoreAsk Dr. Giles: 6 month scan showed a very small dot, I’m worried.
Jean writes: I am a healthy 44 year old female with two great sons ages 13 and 22. I had liver resection surgery with Dr Stuart Knechtle at UW Madison, WI in April 2008 due to a large tumor on my liver. Path reports stated it was contained to the liver, no lymph node involvement,…
Read MoreAsk Dr. Giles: I’m doing well, but the longer I live the more terrified I get.
Sarah writes: Dr Giles, I will be 3 yrs post radiation treatment in November. I was told that I had a 40% chance of living 5yrs. My husband and family are thrilled that I have been doing so well but the longer it is the more terrified I get. I feel like time is running…
Read MoreAsk Dr. Giles: I live alone, every decision is mine alone, it’s frightening.
Roberta writes: I’m 77 YOF, diabetic, with liver CA due to islet cell pancreatic CA 5 yrs ago. I have CA pills to begin this week, am afraid, putting off taking them. I am alone, keep my home alone, 1 son who is out of town a lot, and without emotional support. Where can I…
Read MoreAsk Dr. Giles: How frank should we be with our teenage sons?
Donata writes: Dear Dr. Giles, First of all, I am sorry for the loss of your best friend to cc.My husband was dx. 9/07 & our life outlook has changed to say the least. I mean he has a wonderful attitude, positive ect. however I have a question regarding our 2 boys. Ages are 12…
Read MoreAsk Dr. Giles: Am I just existing? Will it ever get better?
Betty writes: Am I just existing? Will it ever get better? On April 10th, it will be 1 yr. and 8 months since losing Sam. I feel like I am doing everything possible but recently two of my closest friends suggested that I get some professional help. The reason that this got my attention is…
Read MoreAsk Dr. Giles: I am having problems trying to help my husband feel steady
Michele writes: My husband is just coming to terms with the fact that he will not go on much longer. Maybe days/weeks. He is obviously very depressed ( this was a shock as he really expected to go through many years of treatments like Jeff G.) Am having problems trying to help him feel steady,…
Read MoreAsk Dr. Giles: I don’t know how to balance it all. Any suggestions?
Cindy writes: I am a mental health counselor in SC. My mother-in-law has cholangiocarcinoma, inoperable. She is 76. My husband is an only child and is trying to balance work and caring for his mother 3 1/2 hours away. His father is partially blind and deaf, diabetic and very difficult as he seems to be…
Read MoreAsk Dr. Giles: What is the best way to explain to my grandchildren…
Jeff G writes: What is the best way to explain to my grandchildren age 6 and 4 about my disease of CC and that PaPa will be leaving them. I guess timing/when and how. What would be less traumatic? Is simply saying I’m sick and going to heaven to be one of god’s angels? My…
Read More