Monthly Scientific Update – September 2022 (Can bacteria help fight cancer?)
George Riddle, Dr. Reham Abdel-Wahab
Bacteria live in our guts – in all of us. Some of them are good; some are not. Some of them help us digest food; some help fight or promote infections; some work for or against cancer and its treatment. Can they help fight cholangiocarcinoma? Perhaps, if we do it right.
Our doctors and scientists are paying attention. Last February, a session was dedicated to its study at the 9th Annual Conference of the Cholangiocarcinoma Foundation (CCF). As the presentations made clear, gut bacteria are increasingly recognized as critical to cancer diagnosis and progression. Hopefully, the bacteria will also lead to a new and exciting role in treatment.
Immune therapy, which is becoming part of cholangiocarcinoma treatment, seems to be especially influenced by gut bacteria. Dr. Sivan and his group at the University of Chicago noticed that mice from one supplier responded much better to PD-1 blockers than mice from another supplier. They traced the different responses to different bacteria in the mouse intestines due to the food and conditions where they were raised. Mice with gut bifidobacterium responded well; those with lactobacillus experienced no improvement.
Clostridium butyricum, known as CBM-588, is a remarkable example. It is a bacterium found in Japanese soil discovered by Dr. Miyairi. This probiotic has been available as an over-the-counter stomach remedy in Japan and much of Asia since the 1940s. It is also approved as a supplement in the European Union.
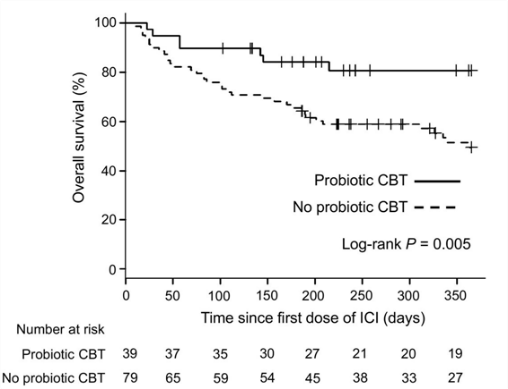
CBM-588 has been studied for its medical effects, especially with immune therapy. It seems to help. Drs. Tomita and Ikeda at Kumamoto University Hospital in Japan led a group that treated more than 100 lung cancer patients with PD-1 blockers. They realized that gut bacteria can have beneficial effects on immune therapy and were interested in seeing whether CBM-588 might enhance the PD-1 blockers; they checked these patients’ medical records. They found that 39 of the 118 patients had also been taking CBM-588. The patients taking CBM-588 had notably longer survival, with a median progression-free survival of 250 days vs. only 101 days for patients who did not take CBM-588. The median overall survival was not reached among those on CBM-588 but was only 361 days among those not on CBM-588 (see chart).

Dr. Tomita’s group noted that proton pump inhibitors – PPIs, like Prilosec – and some antibiotics could reduce the efficacy of PD-1 blockers. They found that in this small sample of patients, taking CBM-588 along with PPIs restored the effectiveness of PD-1 blockers in patients without PPIs. Among patients taking both PPIs and antibiotics, patients also taking CBM-588 had much longer survival (> 1 year vs. 79 days).
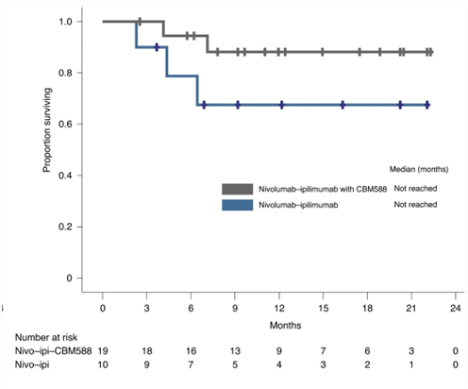
Other groups have been studying c. butyricum, too. Drs. Dizman and Meza at Yale University and City of Hope and their groups undertook a Phase-1 controlled trial of the addition of CBM-588 to the regular PD-1 blocker (nivolumab) and CTLA-4 blocker (ipilimumab) against kidney cancer. They, too, found that patients given the CBM-588 along with the immune therapy did much better, with a median progression-free survival of 12.7 months vs. 2.5 months and a much better 12-month overall survival (see chart). CBM-588 is currently in trial, combined with chemo and immunotherapy against kidney cancer (NCT05122546).
So, we have reason to hope. There are signs that good gut bacteria can significantly improve the efficacy of immunotherapy. But, as Dr. Kelly pointed out in her presentation at the conference, most studies have been retrospective and are therefore subject to confounding influences. Most have had small numbers of patients and are also subject to statistical uncertainty. Not many studies have included biliary cancers. Much work lies ahead.

George Riddle is a CCF Research Advocate Volunteer

Reham Abdel-Wahab is the Cholangiocarcinoma Foundation’s Director of Research and Chief Scientific Officer.
References:
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015 Nov 27;350(6264):1084-9. doi: 10.1126/science.aac4255. Epub 2015 Nov 5. PMID: 26541606; PMCID: PMC4873287.
Stoeva MK, Garcia-So J, Justice N, Myers J, Tyagi S, Nemchek M, McMurdie PJ, Kolterman O, Eid J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. 2021 Jan-Dec;13(1):1-28. doi: 10.1080/19490976.2021.1907272. PMID: 33874858; PMCID: PMC8078720.
Tomita Y, Ikeda T, Sakata S, Saruwatari K, Sato R, Iyama S, Jodai T, Akaike K, Ishizuka S, Saeki S, Sakagami T. Association of Probiotic Clostridium butyricum Therapy with Survival and Response to Immune Checkpoint Blockade in Patients with Lung Cancer. Cancer Immunol Res. 2020 Oct;8(10):1236-1242. doi: 10.1158/2326-6066.CIR-20-0051. Epub 2020 Jul 14. PMID: 32665261.
Tomita Y, Goto Y, Sakata S, Imamura K, Minemura A, Oka K, Hayashi A, Jodai T, Akaike K, Anai M, Hamada S, Iyama S, Saruwatari K, Saeki S, Takahashi M, Ikeda T, Sakagami T. Clostridium butyricum therapy restores the decreased efficacy of immune checkpoint blockade in lung cancer patients receiving proton pump inhibitors. Oncoimmunology. 2022 May 27;11(1):2081010. doi: 10.1080/2162402X.2022.2081010. PMID: 35655708; PMCID: PMC9154751.
Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, Frankel P, Cui Y, Mira V, Llamas M, Hsu J, Zengin Z, Salgia N, Salgia S, Malhotra J, Chawla N, Chehrazi-Raffle A, Muddasani R, Gillece J, Reining L, Trent J, Takahashi M, Oka K, Higashi S, Kortylewski M, Highlander SK, Pal SK. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 2022 Apr;28(4):704-712. doi: 10.1038/s41591-022-01694-6. Epub 2022 Feb 28. PMID: 35228755; PMCID: PMC9018425.