
Webinar: NuTide 121 Clinical Trial
Home / Blog / [wpbb post:title]
NuCana will introduce the science behind NUC-1031, the medicine being compared in combination with cisplatin in the NuTide:121 study against the current standard of care in Biliary Tract Cancer, gemcitabine and cisplatin.
We will also provide an overview of previous study data, which has led to the initiation of the phase III study.
NuCana will further provide an overview of the NuTide:121 study, its aims and objectives and what a patient can expect from participation.
| ClinicalTrials.gov Identifier: | NCT04163900 |
NuCana is a clinical stage bio-pharmaceutical company focused on significantly improving treatment outcomes for patients with cancer. We apply our ProTide technology to transform some of the most widely prescribed chemotherapy agents, nucleoside analogues, into more effective and safer medicines.

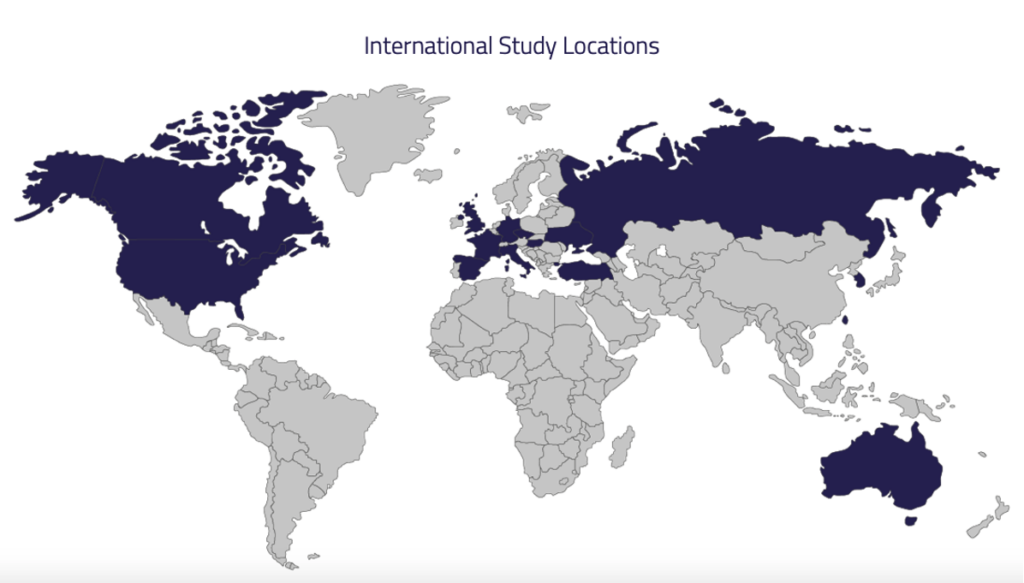
NuTide:121 is currently enrolling patients with histologically or cytologically confirmed adenocarcinoma of the biliary tract (cholangiocarcinoma, gallbladder, or ampullary cancers) that is locally advanced, unresectable or metastatic, and who have not yet received prior systemic treatment for biliary tract cancer.
US, Canada, UK, Spain, France, Germany, Italy, Hungary, Czech Republic, Turkey, Ukraine, Russia, Taiwan, Australia, South Korea